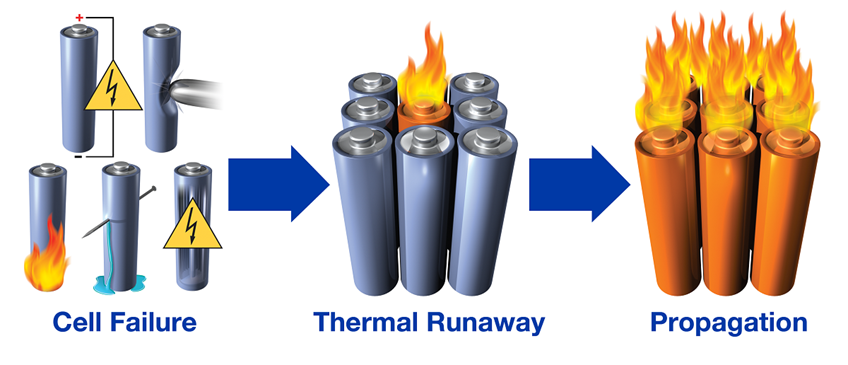
The Threat of Thermal Runaway
If you’ve flown in the last couple decades, you’ve surely been made aware of the danger of lithium-ion batteries aboard aircraft: spontaneous combustion of laptop batteries in the baggage hold, smartphones sandwiched between the seats catching fire (you know, the whole “don’t move your seat, call a flight attendant” spiel). But what about lithium-ion batteries provokes such misbehavior? It actually comes down to physics, chemistry, and one major inherent limitation present in many battery configurations – thermal runaway. In fact, the threat of thermal runaway remains a large safety concern not only for airlines but for a myriad of other industries, including the burgeoning battery electric vehicle sector. But while the unpredictability of a lithium-ion battery catastrophe can instill fear in the average consumer, the chemistry and physics of thermal runaway are not a mystery. In fact, it’s a fairly well understood process. Let’s dive in.
A car battery that underwent thermal runaway. Source: Adobe Stock
The Chain Reaction
First, let’s take a look at the term itself. The “thermal” in thermal runaway refers to heat generated in the chemical reactions taking place. A chemical reaction that generates heat is what chemists call an “exothermic” reaction – one where the process of converting reactants to products by breaking and reforming chemical bonds releases heat. And “runaway”? Well, it just so happens (as is nearly always the case) that the chemical reactions taking place in a thermal runaway event are encouraged and even sped up by heat. As you’ll see in a minute, at a certain temperature threshold – the aptly named “thermal runaway temperature”, the chemical reactions start to ramp up uncontrollably.
So what chemical reaction are we referring to here? Generally, it is the decomposition of the battery’s electrodes, i.e. the cathode and anode materials—the latter an oxide of Lithium, the former usually graphite or a lithium alloy. These decomposition reactions are the exothermic (heat releasing) reactions that drive thermal runaway. And in the presence of excessive heat, the decomposition of the anode and cathode can become self-sustaining, meaning the heat produced by the reaction is sufficient to sustain the reaction itself.
Critical Temperatures
In the world of battery chemistry, engineers like to refer to two critical temperatures: The self-accelerating decomposition temperature (SADT), and the thermal runaway temperature. The SADT refers to the lowest temperature at which the reaction becomes self-sustaining. That is, if left alone, the battery would eventually spiral out of control into some sort of catastrophic combustion. Before this happens, however, the rates of those decomposition reactions are still slow enough for active cooling mechanisms to prevent a catastrophic destruction of the battery — things like venting of excess heat, submersion in an ice bath, etc.
But once you reach the thermal runaway temperature, there is literally no stopping the thermal runaway event. Temperature continues to rise as chemical energy is converted into thermal energy, and once a certain ignition threshold is reached combustion will take place. This will continue to propogate, or spread, from cell to cell until the entire battery goes up in smoke (or flame). For reference, one study found the SADT and thermal runaway temperature for common battery materials (Lithium Cobalt Oxide cathode and Lithium Hexafluorophosphate anode) to be 66.5°C and 75°C1, respectively (152°F and 167°F).
Heat & Short Circuits
Now where might the heat to trigger thermal runaway come from in the first place? Well, the fastest way to get there is creating a short circuit within the battery i.e., a direct, low-resistance path from cathode to anode. This can happen several ways. The most unavoidable is blunt mechanical deformation like complete mangling of the phone in between airplane seats, or as pictured below, puncturing. Perhaps more common yet easier to detect and mitigate are manufacturing defects, as highlighted in the 2006 recall of nearly 10 million Sony laptop batteries or the more recent FAA ban on Samsung Note7 phones on flights after the 2017 recall of a few million units.
But even if we had a perfectly manufactured, puncture-proof battery with no short circuiting, we would still have the problem of heat. Environmental heat is an obvious design consideration here (batteries also happen to perform poorly at colder temperatures as well, due to several different effects). But the heat caused by charging is also one of the main design constraints battery engineers deal with today. And the faster one tries to charge a battery, the more heat there is produced. With the attractiveness and feasibility of electric vehicles being highly dependent on their analogous “time to charge” (as an alternative to combustion engines that take minutes to fuel up), mitigating this heat has become a major focus of battery engineers.
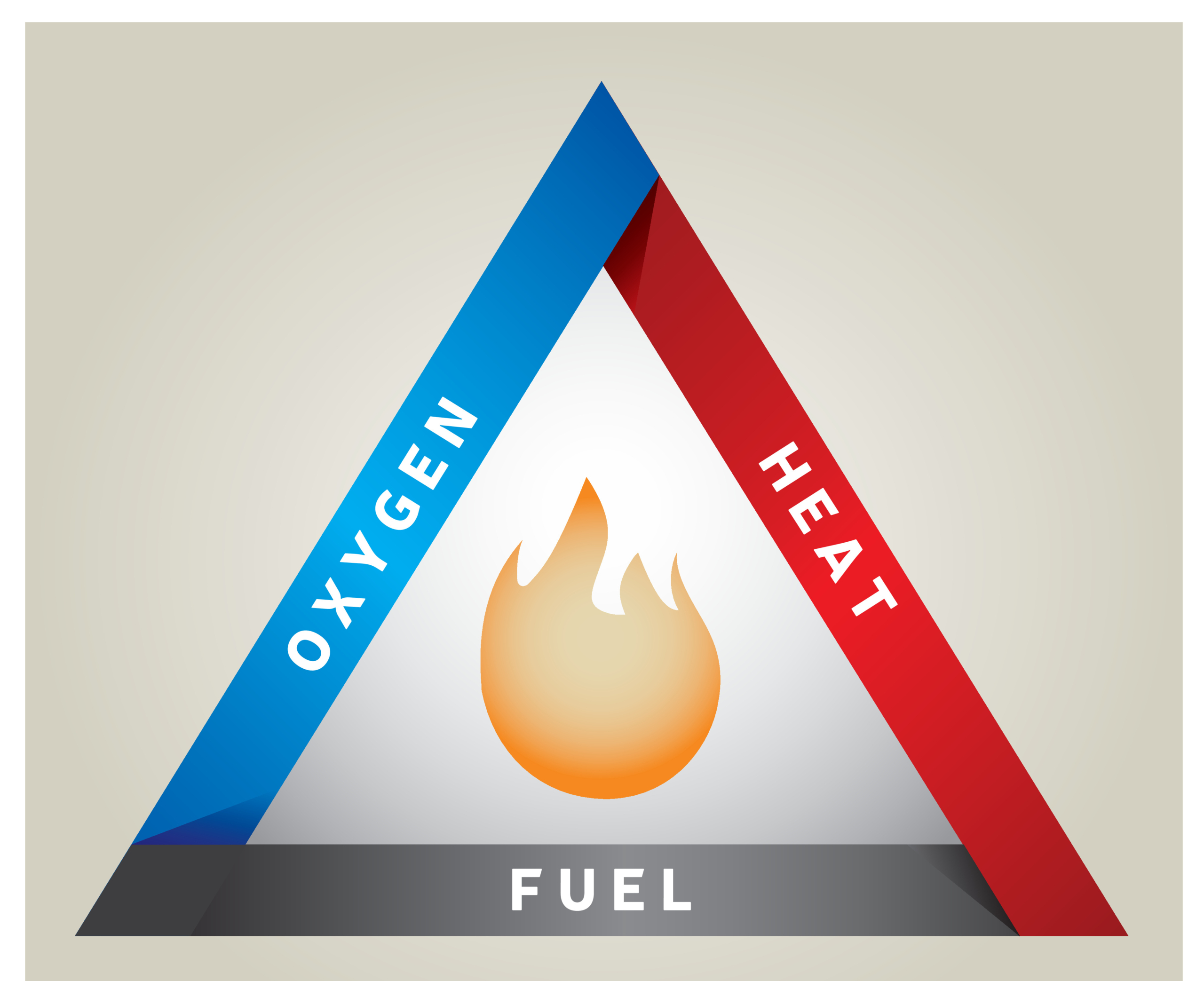
The three necessary components for combustion: fuel, oxygen, and an ignition source. Fuel typically in the form of the flammable liquid/gel electrolyte solution, oxygen from the decomposing cathode (and anode if it is an oxide), and ignition provided by the heat. Source: Adobe Stock
Solving for Thermal Runaway & Fast Charging
A primary focus on thermal management and finding a cooling solution that enables rapid charging has driven engineers at LION Smart to implement a technology called immersion cooling in their development of the LIGHT Battery. Essentially, it meets the high thermal demands of ultra-fast charging (charging times that are on par with fuel-up times of conventional combustion engine vehicles) through a more efficient removal of heat. In principle, immersion cooling actively brings liquid coolant into contact with the individual sources of heat – the cells themselves. And while it brings with it many new design challenges, it promises to make rapid charging a reality and electric mobility more feasible than ever before.
If you’d like to hear more about the how LION Smart is making rapid charging a reality, check back soon for the next article on immersion cooling and thermal management.
Authors:
Wyatt Boyd
Arne Siegner